Key points
- The periodic table arranges all the chemical elements in a specific way.
- Patterns are visible in the way that elements behave, and this allows us to make predictions about other elements.
Periodic table activity
Play this game to learn about lots of different elements in the periodic table.
In the periodic table, what terms are used to describe a vertical column and horizontal row?
A vertical column is called a group, and a horizontal row is called a period.

Video - Group 1 elements
Watch this video all about the group 1 elements The alkali metals. of the periodic tableA table which lists all of the chemical elements and arranges them in a way that is useful. It allows us to spot patterns and make predictions about other elements..
While you're watching, listen out for the chemical propertyThe way an element or compound reacts with other chemical substances. and physical propertyA property of an element or compound which can be directly observed or measured. For example, melting point, electrical conductivity, appearance at room temperature. of the Group 1 elements.
Gethin: And today we're going to be looking at the periodic table. Let's take a look at some Group 1 metals then - also known as alkaline metals.
Fran: Group 1 elements are called the alkali metals. They're soft and shiny when freshly cut, but they quickly tarnish as the metal reacts with oxygen in the air. This is because they're very reactive. When a Group 1 element reacts with water it produces a metal hydroxides solution and hydrogen gas. Let's try it with a lithium.
So, on with eye protection and gloves. Here I've got some lithium and I'm just going to cut a little bit off. And do you see how quickly it tarnishes, how quickly it's changing colour? Here we've got some water. And if I put some universal indicator in, like this, you'll see that it's green and that means it's neutral.
But let's see what happens when we put the lithium in.
Well, you can see it's reacting with the water straight away - it's steadily fizzing away there. It's transferring its energy to its surroundings by heating. And can you see the little purple trail there? That universal indicator is showing us that the water now contains an alkali in this case - lithium hydroxide.
So let's try it with another Group 1 metal - sodium.
As you can see it reacts far more vigorously then lithium.
Why is that?
Well, sodium is below lithium in group one and the reactivity of the alkali metals increases as you go down the group, so potassium is even more reactive.
Wow, look at it.
[Crackle]
Gethin: It's fair to say that you love the Group 1 metals, don't you.
Miss Armit: They are very cool. As you can see the metal's get increasingly more reactive as you go down this group. We saw there lithium, then sodium and then potassium, but you didn't see rubidium, caesium and francium.
Gethin: Francium. That's not an element that you hear a lot of.
Miss Armit: No, it's the second rarest element on Earth. So, the reason we didn't see the reactions of those last three metals is that they are so reactive the explosion would be far too big.
Gethin: So, the further down the table you go the more reactive they become.
Can you remember the chemical and physical properties listed in the video of the Group 1 elements?
- Soft
- Shiny when cut
- Tarnish quickly
- Very reactive
Periodic trends
Groups
elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. are grouped together into columns going vertically down the periodic table, called groupElements in a group are in the same vertical column going down in the periodic table. They have similar properties.. Elements in the same group have similar chemical properties, and there is usually a pattern in their physical properties too.
For example, the elements in group 2 share a similar chemical property in that they are all metalA substance which has the typical properties of a metal. Metals are found to the left and in the middle of the periodic table. which react with oxygen. As you go down group 2, the metals become easier to melt. This is a pattern of a physical property.
Periods
Elements grouped together going horizontally across are called periodElements in a period are in the same horizontal row going across in the periodic table. . It is often harder to see a pattern going across a period.
Sometimes the patterns in the periodic table are called periodic trendA change in properties that can be predicted using the periodic table.. A trend is another word for a pattern.
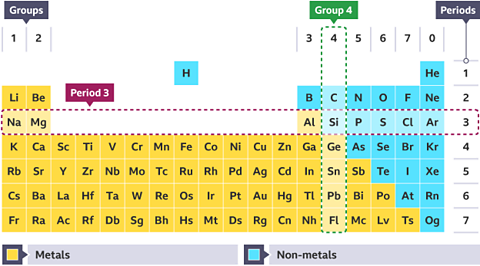
Identify the highlighted period and group for the periodic table below.
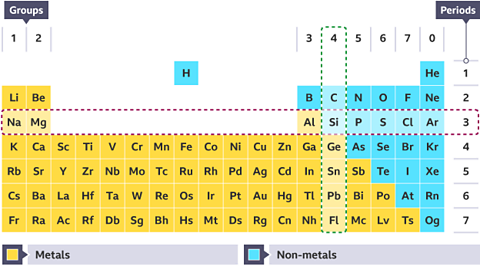
The table shows:
- Group 4 going down vertically
- Period 3 going across horizontally
Group 1 elements
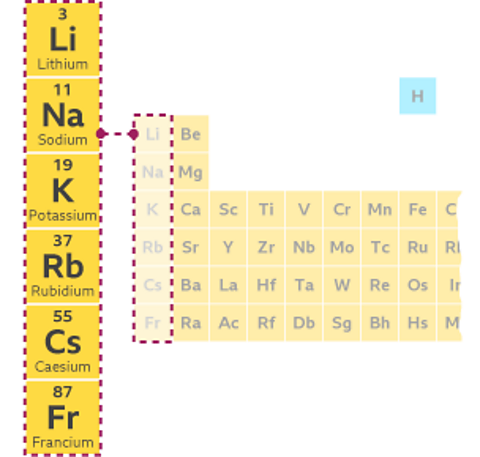
The Group 1 elements are called the alkali metals.
They include lithium, sodium and potassium, and are placed in the first vertical column on the left of the periodic table.
All the Group 1 elements are very reactive, and all react with water to produce an alkaline solution.


Did you know?
Group 1 elements have to be stored under oil to ensure air including water vapour can’t react with them. This is because Group 1 metals are very reactive.

Making predictions about reactivity
The specific arrangement of Group 1 elements on the periodic table allows us to make predictions about their reactivity. For example:
- Lithium (Li) reacts quickly with water. It floats and produces bubbles. It moves slowly around the surface of the water.
- Sodium (Na) is the next element and is more reactive than lithium. When it reacts with water it also floats, but it moves faster around the surface. It produces bubbles more quickly than lithium.
Using the periodic trend of group 1 elements, can you predict how a small piece of potassium (K) will react when it is added to water?
When potassium (K) is added to water, the metal floats and fizzes violently. It melts and moves around very quickly on the surface of the water. The metal catches on fire, and some sparks are produced.
Therefore we can conclude that:
The reactivity of Group 1 elements increases as you go down the group.

Group 7 elements
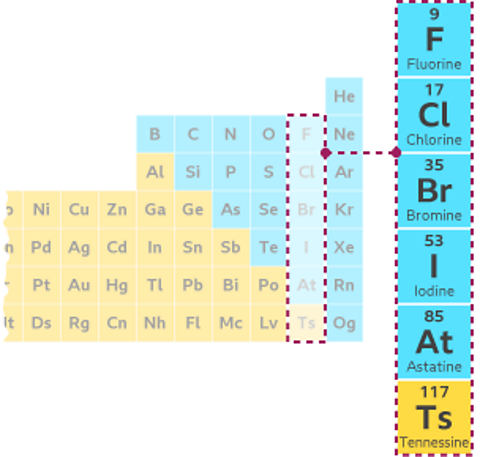
The Group 7 elements are mostly non-metalA substance that has the typical properties of a non-metal. Non-metal elements are on the right hand side of the periodic table., known as the halogens. They include the elements chlorine, bromine and iodine.


Non-metal elements are on the right of the periodic table. Metal elements are found on the left and in the middle of the table.

Did you know?
There are still some elements that scientists haven’t yet found in nature or managed to make in laboratories. We only know they exist because of patterns in the periodic table.

Making predictions about physical properties
The interesting trend in melting and boiling points of Group 7 elements allows us to make predictions about their physical properties.
What does this graph show us about the melting and boiling points of halogens?
It shows us that the melting and boiling points increase going down the group, which affects their state at room temperature.
Video - Groups 7 and 0
Watch this video about the Group 7 and Group 0 elements.
Listen out for as many chemical and physical properties of each group as you can.

Did you know?
Chlorine is a Group 7 element which is a green gas and is frequently used to sterilise drinking water.

Test your knowledge
Quiz
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on Periodic table
Find out more by working through a topic
- count5 of 6

- count6 of 6

- count1 of 6

- count2 of 6
