Key points
- Diffusion is the movement of a substance from an area of high concentrationA large number of particles of one substance in a specific volume of another substance. to an area of low concentrationA small number of particles of one substance in a specific volume of another substance..
- Diffusion occurs in liquids and gases when their particleA single piece of matter from an element or a compound, which is too small to be seen. Particles can be atoms, molecules or ions. collide randomly and spread out.
- Diffusion is an important process for living things - it is how substanceMatter made of a fixed ratio of atoms with characteristic properties. move in and out of cells.
If someone sprays deodorant from the other side of the room, how does the smell reach your nose?
Through a process called diffusion. The deodorant particles bump into air particles and are spread throughout the room. We smell the deodorant particles that reach our noses.
Video - diffusion
Watch this video to find out how the movement of particles in gases and liquids causes diffusionWhen a substance spreads out from an area where there are a lot of particles to an area where there are fewer particles. Diffusion occurs because gas and liquid particles move at random. .
While you are watching, think about why diffusion is different in solids, liquids and gases.
How does the movement of particles and gases cause diffusion?
Gethin : OK, Miss Armit, what exactly is the particle model.
Miss Armit: The particle model as a way of explaining the way particles move and collide. There's four things you need to know about it. All substances are made of particles. The particles are attracted to each other - some more than others. And the particles are constantly moving with what's called 'Kinetic Energy'. As temperature increases, this kinetic energy increases so they can move around more.
Gethin : OK. Those are the four things to remember. Now, substances are made up of solids, liquids and gases. Do the particles move around in the same way in each?
Miss Armit: No, not quite. They move round differently in each one. So in gases they are far freer to move around than in liquids and in solids. They have a lower density and they're far apart. In solids they're much closer together and they can only vibrate. And liquids are kind of in between. Their particles are spread out randomly and their density is less than the solids, but higher than gases.
VOICEOVER: The particles that make up gases and liquids move randomly. This movement allows the action of diffusion to happen.
For example, if you spray some deodorant in your bedroom. The particles move from an area of high concentration, the place where you sprayed it, to an area of lower concentration - your bedroom doorway. Then, through the rest of the house. The perfume particles have diffused.
Gethin : I got all excited because I thought Miss Armit brought in sweets and balloons for a bit of a party. But that's not the case, it's actually for an experiment. But, how and why have you brought them in? And what's it got to do with the particle model?
Miss Armit: Well, articles are too small to see. So in order to demonstrate the model we use these to help demonstrate it, so we can see them in different states of matter.
Gethin : What about diffusion? Can happen in all states? And by that I mean solids, liquids and gases?
Miss Armit: So, it happens mainly liquids and gases. This is because the particles can freely move around. It doesn't really happen in solids as the particles are in this fixed position and they can only vibrate on the spot.
Gethin : Alright. And have you got an example of that in front of you, then?
Miss Armit: Yeah, just here. I poured some water on this plate earlier. And this is before and after. So, what happened is the food colouring on the outside of the sweets has dissolved into the water. And then it's moved from the high concentration of food colouring around the sweet to the lower concentration in the middle. And you can clearly see the diffusion is illustrated.
Why does diffusion happen faster in gases than in liquids?
The particles in gases have more energy and are more widely spaced out than the particles in a liquid. This means that gas particles will bump into each other harder and more often. Which makes gas particles spread out more quickly than liquid particles can.
What is diffusion?
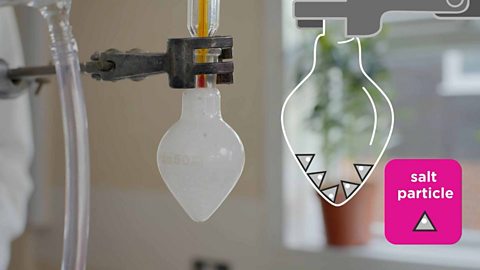
Diffusion is the process by which particles of one substance spread out through the particles of another substance. Diffusion is how smells spread out through the air and how concentrated liquids spread out when placed in water.
Diffusion happens on its own when the particles spread out from an area of high concentrationThe number of particles of one substance in a specific volume of another substance., where there are many of them, to areas of low concentration where there are fewer of them.

dissolveThe process when a solute is mixed with a solvent and the solute breaks into much smaller particles and spreads out. is not the same as diffusion. Dissolving is the term used to describe when a substanceMatter made of a fixed ratio of atoms with characteristic properties. breaks up and mixes thoroughly with a solvent to produce a solution.
Slideshow
Click through the slideshow below to see an example of diffusion.

Image caption, In the video, the coloured sweets were arranged on a plate, and then some water was added. What happened next?
Image caption, The colours have spread out away from the sweets. This shows how the coloured particles on the sweets have dissolved and then diffused through the water.
1 of 2
How does diffusion happen?
Diffusion occurs in gases like air and liquids like water because their particles can move around and collide with each other randomly.
For example, if you mix two drinks, the liquids diffuse into each other. Blackcurrant squash has a high concentration level. When the squash is mixed with water, it becomes less concentrated and is diluted.
Watch the video below to find out how making a cup of coffee involves diffusion.
Video
Find out how diffusion is involved in the coffee-making process
Making a good coffee really is a science. Every coffee that we put on our menu has to run through its own experiment. We're trying to find the best tasting recipe for that coffee and the experiment has to be reliable.
Diffusion is essential to the coffee-making process. Diffusion is the movement of a gas or liquid from an area of high concentration to an area of low concentration.
We start with a whole bean, which we then crush or grind, the powder is then put into something that we call a portafilter. Then we're compressing that powder, we're packing it down really hard with lots of pressure so that the water can run through slowly and diffuse within the coffee, that's eliminating air bubbles and ensuring that there is a lot of contact time with that water, giving the coffee the best chance to diffuse.
My favourite thing about my job is to be able to transform someone's day from a bad one to a good one with just a nice coffee, and it's all thanks to science.
Summary
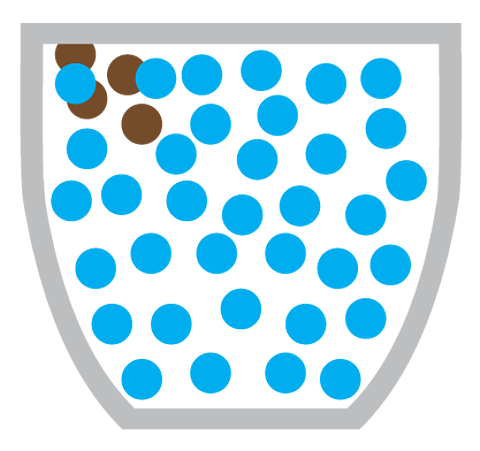
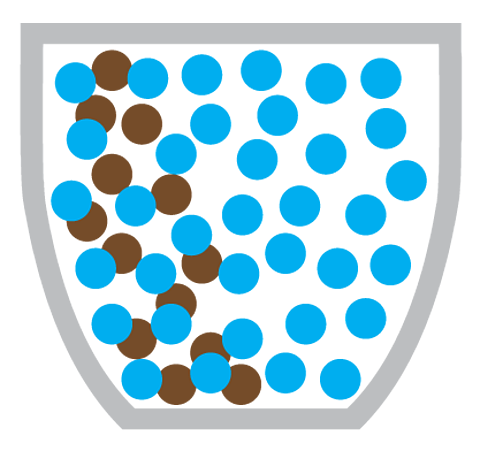
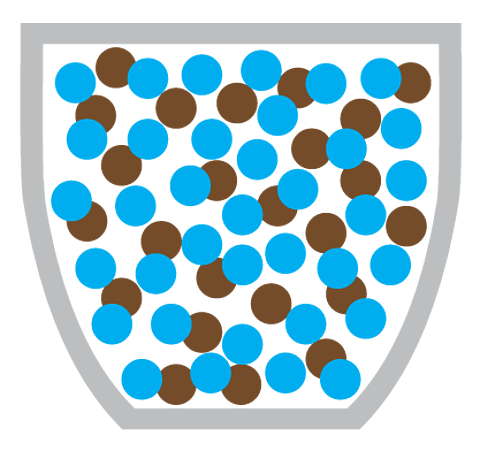
Why doesn’t diffusion happen in solids?
Diffusion doesn’t happen in solids because the particles in a solid cannot move around, instead they vibrate about a fixed position.
What affects diffusion?
Two factors affect diffusion:
The type of substance - substances diffuse more quickly through gases than through liquids. This is because the particles in a gas are more spread out and move faster than the particles in a liquid.
Temperature - the hotter the gas or liquid, the faster diffusion happens. This is because the particles have more energy and move faster at higher temperatures.
Working scientifically
Working safely in the lab
An investigation into diffusion could be conducted by adding \(7cm^3\) copper sulfate solution to \(50cm^3\) cold water and also to \(50cm^3\) hot water.
What safety precautions would you need to take before carrying out this investigation?
Make sure that you know the laboratory safety rules and take precautions to reduce risks.
Make sure you have researched any potentially hazardous chemicals. Hazard symbols give information about chemicals and materials.
You might need to find out more about copper sulfate from a textbook, data book or reliable website.
Find out more about working safely in science.
Test your knowledge
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on Pure and impure substances
Find out more by working through a topic
- count7 of 7

- count1 of 7
- count2 of 7
- count3 of 7
