The Earth’s atmosphere

The Earth’s atmosphere is the relatively thin layer of gases that surround the planet. It provides us with the oxygen we need to stay alive and is approximately 80 km thick.
Air is the mixture of gases that makes up the atmosphere.
The amount of water vapour in the air varies from place to place, and day to day. For this reason, the proportions of the gases in the air are usually given for dry air.
Some of the gases in the air are elements:
- nitrogen, ±·в‚‚
- oxygen, °їв‚‚
- argon, Ar
Some are compounds, including:
- carbon dioxide, C°їв‚‚
- water vapour, ±бв‚‚O
| Gas | Symbol | % of gas in dry air |
|---|---|---|
| Nitrogen | ±·в‚‚ | 78 |
| Oxygen | °їв‚‚ | 21 |
| Argon | Ar | 0.9 |
| Carbon dioxide | C°їв‚‚ | 0.04 |
| Other Gases | 0.06 |
This information can be represented in a pie chart:
How oxygen was discovered - Joseph Priestly
Oxygen was discovered in 1774 by Joseph Priestley. He used a 12 inch (30 cm) "burning lens" to focus the sun’s rays and heated mercuric oxide. He noticed that “a most remarkable gas was emitted”.
In this gas he found that a candle burned more brightly, and a piece of red-hot wood crackled and burned brightly and quickly, throwing sparks in all directions.
To complete his experiments, he put a mouse into the new gas in a box. In the same quantity of air, the mouse would have died in about a quarter of an hour, but it lived for an hour, before being removed alive.
Priestly had discovered oxygen gas. However, it was the French chemist Antoine Lavoisier, working at the same time as Priestly, who first named the gas, “oxygen”.
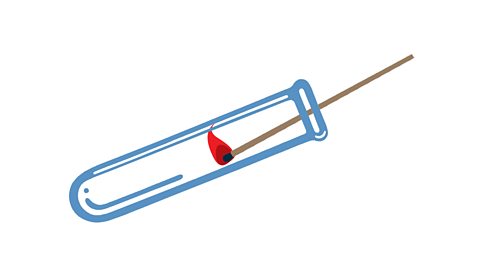
Tests for the gases in air
Oxygen
A glowing wooden splint relights in a test tube of oxygen


Carbon dioxide
Turns limewater from colourless to milky

Nitrogen, carbon dioxide and argon
A lighted wooden splint goes out in a test tube of any one of these gases
Making oxygen

Oxygen can be made from hydrogen peroxide, which decomposes slowly to form water and oxygen. The word equation is:
hydrogen peroxide в†’ water + oxygen
This can be quite a slow decomposition but can be speeded up by adding a catalyst.
A catalyst is just a substance that changes the speed of a chemical reaction without changing the products of the reaction.
A catalyst:
- speeds up reactions
- is not used up during the reaction (its mass is the same at the start and end of the reaction)
- is chemically unchanged after the reaction has finished
Usually, only a very small amount of catalyst is needed, and different catalysts are needed for different reactions.
In this case, a black powder, manganese(IV) oxide, is added as the catalyst. When manganese(IV) oxide is added to hydrogen peroxide, bubbles of oxygen are still given off, but more quickly.
Apparatus and chemicals
- Conical flask with side arm, thistle funnel, delivery tube
- 2 test tubes and 2 stoppers
- Basin/trough of water
- Test tube rack
- Trough half-filled with water
- Bee hive shelf to stabilise test tubes
- Manganese(IV) oxide - about 3 g
- Hydrogen peroxide solution
- Small beaker
- Wooden Splint
MethodCarefully place the manganese (IV) oxide into the conical flask.
Collect 50 cm3 of hydrogen peroxide solution in a small beaker.
Connect the delivery tube and thistle funnel to the conical flask with the delivery tube in a basin of water. (As in the diagram above)
Have three test tube filled with water also inverted in the trough of water.
Carefully pour the hydrogen peroxide solution into the thistle funnel. Wait 5 s then carefully place the first water filled test-tube over the end of the delivery tube.
You will see bubbles of gas rising through the water. Allow the test tube to fill with gas.
Leave the test tube inverted in the water to retain the gas.
Repeat until three test tubes of gas have been collected.
Remove each test tube from the water as required for the following tests.
Testing the gas

To the first test tube insert a glowing splint – record your observations.
To the second test tube insert a burning splint – record your observations.
To the third test tube add three drops of universal indicator – record your observations.
Conclusion
The test for oxygen is that it relights a glowing splint (Test one above)
Oxygen makes things burn more vigorously and is a neutral gas. (Tests two and three above)
Uses of oxygen
- Oxygen is used in medicine to help patients with breathing difficulties
- Oxygen is a crucial treatment for many patients with severe Covid-19, since the disease affects the lungs’ ability to work properly
- To cut and weld steel - acetylene gas is mixed with oxygen to increase the burn temperature to about 3000 degrees
A short video explaining how oxygen can be used to help the body.
Combustion Reactions
What is a combustion reaction?
When some substances react with oxygen, they catch fire. This is called burning or combustion.
A combustion reaction releases energy to its surroundings and is called an exothermic reaction. This is mostly heat energy, but light energy and sound energy are also released. Note that some other reactions are endothermic reactions – they take in energy from their surroundings.An example of a combustion reaction is when methane is burned in a Bunsen burner.

As combustion is the reaction of a substance (called a fuel) with oxygen, then it is obvious that oxygen must be present for combustion to take place and for us to see fire. Normally, the oxygen required in combustion reactions comes from air.
Fire needs three things:
- a fuel
- oxygen
- heat
The fire triangle shows the three things needed for a fire to start and keep going.
If one of the sides of the fire triangle is removed, a fire will not start, and a fire that is already burning will go out. Firefighting relies on this principle. The fire will go out when the fuel runs out, but it is often unsafe to leave a fire that long. Different types of fires need to be tackled in different ways.
| Fire | How to put it out | Part removed |
|---|---|---|
| Chip pan (oil) fire | Cover the pan with a lid, damp cloth or tea towel. Never pour water on an oil fire! Oil floats on water and the fire will spread out further | Oxygen |
| Forest fire | Make a fire break (cut down a line of trees) | Fuel |
| Wood or paper fire | Spray with water | Heat |
| Petrol fire | Cover with sand. Never pour water on an petrol fire! Petrol floats on water and the fire will spread out further | Oxygen |
| Electrical fire | Spray with a carbon dioxide fire extinguisher. Never pour water on an electrical fire! because water conducts electricity and you could be electrocuted | Oxygen |
Complete combustion
Many of the fuels we use contain carbon and carbon compounds called hydrocarbons. Coal, oil and natural gas are widely used fuels and when they burn, the hydrocarbons react with oxygen. If there is plenty of air, complete combustion happens:
- the hydrogen atoms combine with oxygen to make water vapour, ±бв‚‚O (hydrogen oxide)
- the carbon atoms combine with oxygen to make carbon dioxide, C°їв‚‚
- the maximum amount of energy is released
The word equation for the combustion of any hydrocarbon is:
hydrocarbon + oxygen в†’ water + carbon dioxide
It is worth remembering that a hydrocarbon is burned to release energy, not to produce water and carbon dioxide!Natural gas is mostly the hydrocarbon methane, CHв‚„. Here is the word equation for its complete combustion:
methane + oxygen в†’ water + carbon dioxide
Candles are made from paraffin wax, a hydrocarbon. The diagram shows how they can be used in the laboratory to investigate combustion.
The carbon dioxide produced is tested using limewater. This turns milky (cloudy white) when carbon dioxide is bubbled through it.
Test for Water
The water can be tested by using putting anhydrous copper (ii) sulfate powder in U-tube and it will turn blue.
Incomplete combustion
If there is not enough air or oxygen for complete combustion, incomplete combustion happens instead. Water vapour and carbon dioxide may be still produced, but two other products are also produced:
- carbon monoxide, CO
- particles of carbon, which appear as soot and smoke
Problems with incomplete combustion
Carbon monoxide is a colourless, toxic gas with no smell. It binds to haemoglobin in your red blood cells, preventing them from carrying oxygen to the cells in your body. This deprives the heart, brain and other vital organs of oxygen. Large amounts of carbon monoxide can overcome you in minutes without warning — causing you to lose consciousness and to suffocate.

As carbon monoxide is colourless and has no smell, it is very difficult to tell if you are breathing it in. It can be detected by electronic detectors which are often fitted near to boilers and gas fires.
Particles of carbon in soot and smoke can also cause health problems for humans because they irritate the lining of the lungs, can make asthma worse, and perhaps even cause cancer.

Oxidation
Combustion is an example of a type of reaction called oxidation. In an oxidation reaction, a substance gains oxygen. Metals and non-metals can take part in oxidation reactions.
Metals
Metals react with oxygen in the air to produce metal oxides.
metal + oxygen в†’ metal oxide
For example, magnesium reacts with oxygen to produce magnesium oxide, a white powder, when it is heated in air:
magnesium + oxygen в†’ magnesium oxide

The reaction is even faster and brighter if the magnesium burns in pure oxygen.
Hazard
DO NOT LOOK DIRECTLY AT THE BURNING RIBBON. The burning magnesium ribbon can produce light of sufficient intensity to cause temporary loss of sight. Avoid looking directly at the light source.
Magnesium burns brightly in air and is used in distress flares.
Metal oxides produced in oxidation reactions are bases, they react with acids and neutralise them. Some metal oxides dissolve in water to produce alkaline solutions.
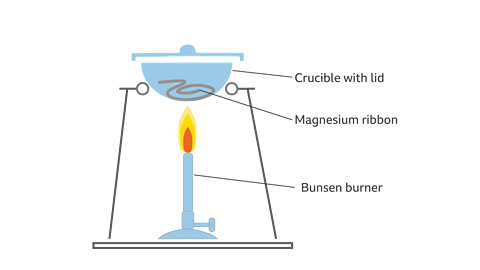
Method
- Put a sample of clean magnesium ribbon into a crucible and put the lid on.
- Measure the mass of the crucible, lid and magnesium, using a top pan balance. Record the mass in a suitable table.
- Strongly heat the crucible over a Bunsen burner for several minutes as shown in the diagram. Use a roaring flame.
- With tongs, carefully lift the lid from time to time to allow sufficient air into the crucible for the magnesium to fully burn, without letting any magnesium oxide escape.
- Continue heating until the magnesium stops glowing when the lid is lifted.
- Turn off the Bunsen burner and allow the apparatus to cool completely.
- Measure and record the mass of the crucible, lid and contents again.
- Calculate any difference in mass
Hazard
The apparatus becomes very hot! You could burn your fingers if you do not take proper care.
- Only lift the lid of the crucible using tongs
- Wait until the crucible has fully cooled and wear heat proof gloves before placing it again on the top pan balance
- Make sure the crucible sits down firmly in the pipe clay triangle which sits on the tripod
- As you are using a Bunsen burner, you must wear safety goggles
Conclusion
The mass of crucible, lid and white powder after heating is greater than the mass of crucible, lid and magnesium before heating.
The magnesium has taken something from the air when it forms the white powder. It has gained oxygen, and this has caused the increase in mass. This is why this is called an oxidation reaction.
Non-metals
Non-metals react with oxygen in the air to produce non-metal oxides.
non-metal + oxygen в†’ non-metal oxide
Here are two examples for the non-metals carbon and sulfur.
Carbon reacts with oxygen to form carbon dioxide:
carbon + oxygen в†’ carbon dioxide
Sulfur burns in oxygen to form sulfur dioxide:
sulfur + oxygen в†’ sulfur dioxide
Non-metal oxides are acidic ( they are only acids when dissolved in water) – they react with bases and neutralise them.
Summary
Many metals and non-metals react with oxygen in the air when they are heated to produce metal oxides and non-metal oxides.The table shows three of these reactions in detail.
| Element | Type | Reaction type | Oxide | Nature |
|---|---|---|---|---|
| Magnesium | Metal | Highly exothermic (it releases energy to its surroundings) - magnesium burns with bright white flame | Magnesium oxide, MgO – a solid white powder | Basic |
| Carbon | Non-metal | Exothermic (it releases energy to its surroundings) - carbon glows orange when heated strongly | Carbon dioxide, C°їв‚‚ – a colourless gas with no odour | Acidic |
| Sulfur | Non-metal | Exothermic (it releases energy to its surroundings) - burns slowly with a blue flame | Sulfur dioxide, S°їв‚‚ – a colourless gas with a sharp, choking smell (the smell of a just-struck match) | Acidic |
Carbon dioxide
Structure and properties
Carbon dioxide is only 0.04% of air, but it is vital to all living things. At room temperature it is a colourless and odourless gas, and it is soluble in water. Green plants need carbon dioxide for photosynthesis, the process that produces glucose and replaces oxygen ). Without carbon dioxide, plants wouldn’t grow, animals would not have food and would die, and without vegetables and meat to eat, we would not survive.
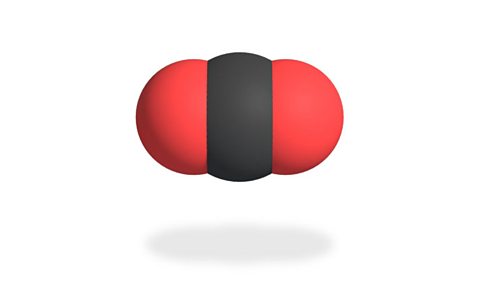
The carbon dioxide molecule is made up of one carbon atom joined to two oxygen atoms. This means it has a chemical formula is C°їв‚‚.

Making carbon dioxide
Carbon dioxide is produced whenever an acid reacts with a carbonate. This makes carbon dioxide easy to make in the laboratory. Calcium carbonate and hydrochloric acid are usually used because they are cheap and easy to obtain.
calcium carbonate + hydrochloric acid в†’ calcium chloride + water + carbon dioxide
Carbon dioxide is slightly soluble in water and denser than air, so one way to collect it is in a dry, upright gas jar, as shown below. As the carbon dioxide falls out of the delivery tube and into the gas jar, it pushes the less dense air out of the top of the gas jar.

The test for carbon dioxide is that it turns limewater milky.
Carbon dioxide is a weakly acidic gas. It turns blue litmus paper red and universal indicator light green.
Carbon dioxide will put out a burning taper.
Uses of carbon dioxide
- Carbon dioxide is soluble in water. When it dissolves it forms a carbonic acid.
Carbon dioxide + water в†’ carbonic acid
Carbonic acid is what gives fizzy drinks their bubbles. At high pressures, more carbon dioxide dissolves in water, and because this reaction is reversible, when the pressure decreases carbon dioxide is released again. This explains why bubbles appear in a bottle of sparkling water or cola when you unscrew the lid.
Green plants need carbon dioxide for photosynthesis
Carbon dioxide will put out a burning taper. It is denser than air, and so it sinks, which means that it can smother a fire, removing the oxygen side of the fire triangle and putting it out. The carbon dioxide from a fire extinguisher is also very cold, which helps to remove heat from a fire too. This is why many fire extinguishers contain carbon dioxide gas.

Carbon dioxide in the atmosphere
The amount of carbon dioxide in the atmosphere is maintained through a very careful, delicate balance.
Processes that increase the amount of C°їв‚‚ in the air include:
- combustion of fossil fuels such as coal, oil and natural gas
- respiration of living things
Processes that decrease the amount of C°їв‚‚ in the air include:
- photosynthesis by plants which converts carbon dioxide and water to make their own food, glucose
- dissolving in sea water
Uses of the gases in air
The greenhouse effect
Without the greenhouse effect the mean temperature on Earth would be about -18В°C. That would make it too cold to support life as we know it.
Greenhouse gases present in the atmosphere include:
- water vapour
- carbon dioxide
- methane
Carbon dioxide, and the other greenhouse gases, in the atmosphere allow heat from the sun to reach the Earth but stops some of the Earth’s heat escaping again. This warms the lower atmosphere.
Increasing carbon dioxide levels
Humans burn fossil fuels such as coal, oil and natural gas, to power cars and other machines, to generate electricity, and to keep our homes and schools warm. Waste gases are released during combustion of these fuels, including carbon dioxide. As the human population increases, more fuel is used, and more carbon dioxide is released.
Global warming
Extra carbon dioxide in the atmosphere increases the greenhouse effect. More heat energy is trapped by the atmosphere, causing the planet to become warmer than it would be naturally. This increase in the Earth’s temperature is called global warming.
The majority of climate scientists agree that there is a link between the increasing levels of carbon dioxide and the increasing temperatures. Global warming is having an effect on the world’s climates.
The impact of climate change
The weather includes the wind, sunshine and rain you see from day to day. The climate is the pattern of weather seen over years and decades.
If there is global warming some of the effects are predicted to be:
- average temperature to increase by 3 В°C or more by the end of the century
- ice melting faster than it can be replaced in the Arctic and Antarctic
- the oceans warming up – their water expanding and causing sea levels to rise
- more droughts and heatwaves
- some parts of the world becoming hotter and some wetter
- hurricanes becoming stronger and more frequent
- changes in where different species of plants and animals can live
- increased number of plants and animal becoming extinct
Glacial retreat as evidence for global warming
Burning and air pollution

When fuels are burned, several atmospheric pollutants are produced.Here are some of the pollutants from burning fuels, where they come from and their damaging effects:

| Pollutant | Source | Damage caused |
|---|---|---|
| Carbon dioxide, C°їв‚‚ | Complete combustion of any fuel containing carbon atoms | Contributes to the greenhouse effect |
| Carbon monoxide, CO | Incomplete combustion of any fuel containing carbon atoms e.g. car exhaust fumes | Toxic gas that can suffocate humans and animals |
| Particles of carbon, C (soot) | Incomplete combustion of any fuel containing carbon atoms | These toxic particles can cause breathing issues, including asthma, bronchitis, coronary heart disease, and possibly cancer. Makes buildings black and grimy. |
| Unburned hydrocarbons | Hydrocarbon fuel molecules which have not been oxidised at all, for example, methane | Contributes to the greenhouse effect. When methane leaks into the atmosphere unburned, it contributes more to the greenhouse effect than the carbon dioxide produced by burning it. Unburned hydrocarbons can also contaminate soil and water |
| Sulfur dioxide, S°їв‚‚ | Combustion of a fossil fuel which contains sulfur impurities e.g. burning coal and petrol | Harmful to breathing system and contributes to acid rain – kills fish and damages limestone buildings |
| Nitrogen oxides, NO and N°їв‚‚ | Oxidation of nitrogen from the air inside the engine of a car, lorry, etc e.g. car exhaust fumes | Harmful to breathing system and contributes to acid rain |

Sulfur dioxide as a pollutant
Sulfur dioxide is produced from the small amounts of sulfur contained in most fossil fuels when they are burnt:
sulfur + oxygen в†’ sulfur dioxide
Sulfur dioxide irritates the skin and parts of the eyes, nose, throat, and lungs. There can be inflammation and irritation of the respiratory system, especially during heavy physical activity when we breath harder.Sulfur dioxide also reacts with oxygen in air and rainwater to make acid rain - rainwater that is more acidic than normal. Acid rain is a dilute solution of sulfuric acid. It harms and kills plants and animals, especially those that live in, or near, water. It can make lakes too acidic for fish to live in.
Acid rain can also cause paint to peel, the corrosion of steel structures such as bridges, and weathering of stone buildings and statues. This is worse when the stone used is limestone, causing it to blacken and crumble.

Nitrogen oxide gases as pollutants
Nitrogen is not present in fuels, but the high temperatures and pressures inside a car engine or factory furnace can cause the nitrogen and oxygen in the air to react together to make oxides of nitrogen.
Two of these made inside engines are Nitrogen oxide, NO, and nitrogen dioxide, N°їв‚‚.
These, like sulphur dioxide, are harmful to the breathing system. They also react with rainwater to form nitric acid and add to acid rain.
Reducing air pollution
- Catalytic converters
The exhaust systems of cars are fitted with catalytic converters. These help reduce the release of toxic gases from the exhaust pipe. They contain platinum and rhodium, which act as catalysts.
The reactions in catalytic converters:
- convert poisonous carbon monoxide into carbon dioxide
- convert nitrogen oxides (which cause acid rain) into nitrogen and oxygen
Platinum and rhodium are very expensive metals, but they are spread out very thinly in the catalytic converter – very little is needed and, because they are a catalyst, they are not used up.
Burn fewer fossil fuels – switch to alternative forms of energy such as wind and solar
Use cleaner fuels in car, buses trains and planes - The UK government has plans to improve pollution due to traffic, and is banning the sale of new fossil fuel cars by 2030.
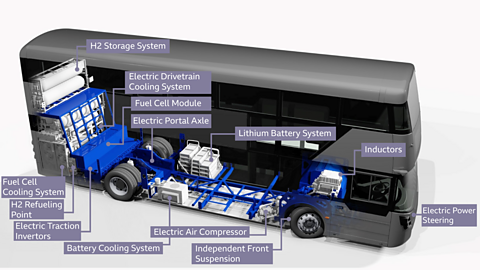
Electric cars and hydrogen fuelled buses are two alternatives to fossil fuel transport. The Ballymena firm, Wrightbus is leading the way in zero-emissions transport, with design and production of the world-first hydrogen double-decker bus. Wrightbus proudly states that; “Designed and built in the UK, our technology is helping the world breathe again”.
- Power stations can filter waste gases in chimneys to remove sulphur dioxide.

- Walk instead of drive
- Use public transport rather than travelling one person to each car
- Use more biomass as fuel
- Turn off lights
- Do not leave electrical appliances on standby mode
More on Chemistry
Find out more by working through a topic
- count8 of 8

- count1 of 8

- count2 of 8

- count3 of 8
