Key Points
- Acids react with most metals.
- When an acid reacts with a metal, the products are a salt and hydrogen.
- This is the general word equation for the reaction:metal + acid в†’ salt + hydrogen
Game - reacting metals with acids
Play an Atomic Labs experiment exploring how metals react with acid.
You can also play the full game
What would the pH of an acidic solution be?
Below 7.
A solution with a pH value which is less than 7 is acidic. The acids which react with metals usually have quite a low pH, of either 3 or less.

Video
Watch this video about the reaction between some metalA substance which has the typical properties of a metal. Metals are found to the left and in the middle of the periodic table. and acidA substance which produces hydrogen ions in solution. Acids have pH values lower than 7. .
While you're watching, listen out for the names of the productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (в†’). made in the reaction.
VOICEOVER: Reactions of metals with acids
Some metals react with acids and when they do the products are hydrogen gas and a solution of the salt.
A salt is a type of compound which is made when an acid reacts with certain metals. The first part of the name of the salt produced comes from the metal and the second part comes from the acid that the metal has reacted with.
Let's look at three metals reacting with three acids. Magnesium plus nitric acid gives us magnesium nitrate. Calcium plus hydrochloric acid gives us calcium chloride. And zinc plus sulphuric acid gives us zinc sulfate. And the salt that we put on our food is produced when sodium reacts with hydrochloric acid, giving us the salt compound sodium chloride.
What are the names of the products made in the reaction between an acid and a metal?
Salt and hydrogen.
Metals and acids
Acids react with some metals to produce a saltA compound which has atoms of metals and non-metals chemically bonded. and hydrogen gas.
Metal + acid в†’ salt + hydrogen
The abbreviation M.A.S.H. can be used to remember this general reaction.

When a metal is put in acid, it gets smaller and smaller as it gets used up in the chemical reactionWhen chemical bonds are broken and made between atoms, so that new substances (compounds or elements) are made..
At the same time, bubbles of gas can be seen. The bubbles produced in the reaction are hydrogen gas.
This can be proven using a burning splint because hydrogen is flammableSomething which is flammable sets on fire easily. . When the burning splint is put into the test tube containing hydrogen gas, a small explosion occurs, making a squeaky pop sound. This shows that hydrogen is present.

What is seen when a metal reacts with an acid?
Bubbles being produced
The metal getting smaller
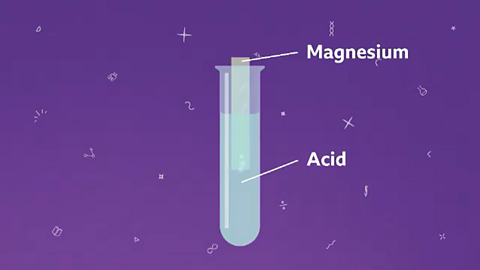
Image caption, A piece of metal like magnesium is added into some acid.
Image caption, Hydrogen gas is produced as the metal reacts with the acid.
Image caption, A salt solution is also formed. The products of this reaction are a salt solution and hydrogen gas.
1 of 3
Reactivity of metals
Some metals are very reactiveThe tendency of a substance to undergo a chemical reaction.. This means they easily take part in chemical reactions to make new substances.Other metals are very unreactive, and do not easily take part in chemical reactions.
If we put the metals in order of their reactivity, from the most reactive down to the least reactive, we get a list called the reactivity seriesA list of elements in order of reactivity, with the most reactive one at the top. The reactivity series usually includes metals and occasionally some non-metals..
True or false?
Gold reacts with acids to produce lots of bubbles.
False!
Gold is not a reactive metal, meaning it does not corrode and is easily malleable over a flame, making it a good element for jewellery making.


Naming the salt from the reaction of a metal and an acid
The name of the salt formed from the reaction of a metal and acid can be worked out using the names of the metal and the acid.
Name of metal + name of acid в†’ salt name
1. The first word is the name of the metal
For example, a salt made when magnesium is added to an acid would have magnesium as its first word.

2. The second word of the name is taken from the name of the acid
Hydrochloric acid в†’ chloride
Nitric acid в†’ nitrate
Sulfuric acid в†’ sulfate

For example, zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen.
The table below has some more examples.
| Metal | Acid | Salt name |
|---|---|---|
| Magnesium | Nitric acid | Magnesium nitrate |
| Calcium | Hydrochloric acid | Calcium chloride |
| Zinc | Sulfuric acid | Zinc sulfate |
The reaction of metals with acids can be described using a chemical equationA way of describing chemical reactions in words or symbols..
A word equation:
magnesium + sulfuric acid в†’ magnesium sulfate + hydrogen
Or a symbol equation:
Mg + Hв‚‚SOв‚„ в†’ MgSOв‚„ + Hв‚‚
What is the name of the salt formed when iron reacts with sulfuric acid?
Iron sulfate.
The word and symbol equation for this reaction is:
Iron + sulfuric acid в†’ iron sulfate + hydrogen
Fe + Hв‚‚SOв‚„ в†’ FeSOв‚„ + Hв‚‚
Activity
Make a set of flash cards for some acids and for some metals.
For the acids use hydrochloric acid, nitric acid and sulfuric acid. For the metals use magnesium, zinc and iron.
- Shuffle the acid flash cards and place them face down in a pile
- Shuffle the metal flash cards and place them face down in a pile
- Take one metal flash card and one acid flash card
- Can you name the salt produced from this reaction?
- Can you write a word equation for this reaction? Remember that hydrogen gas will be produced each time.
How many different salts can you make using your six cards?
| Metal | Acid | Salt name |
|---|---|---|
| Magnesium | Hydrochloric acid | Magnesium chloride |
| Magnesium | Nitric acid | Magnesium nitrate |
| Magnesium | Sulfuric acid | Magnesium sulfate |
| Zinc | Hydrochloric acid | Zinc chloride |
| Zinc | Nitric acid | Zinc nitrate |
| Zinc | Sulfuric acid | Zinc sulfate |
| Iron | Hydrochloric acid | Iron chloride |
| Iron | Nitric acid | Iron nitrate |
| Iron | Sulfuric acid | Iron sulfate |

Test your knowledge
Quiz
Teaching resources
Are you a teacher looking for more resources to support your chemistry lessons? In this short video clip, science presenter Jon Chase uses universal indicator to measure the pH of a solution.
В鶹№ЩНшКЧТіИлїЪ Teach has thousands of free, curriculum-linked resources to help deliver lessons - all arranged by subject and age group.
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on Acids and alkalis
Find out more by working through a topic
- count1 of 3

- count2 of 3

