We know that matter is made up of particles called atoms and that an element is a substance that is made up of only one kind of atom. But what are atoms made up of?
A very important part of chemistry is understanding the structure of atoms and the differences between them.
Around 400 B.C., there was a brilliant philosopher named Democritus, and he proposed that all matter was made of small particles. The Greeks called these particles atomos, meaning indivisible, and the modern word ãatomã is derived from this term.
Structure of the atom
Although Democritus had the right idea, we now know that the atom is not indivisible. Atoms are in fact made up of three smaller particles called protons, electrons and neutrons.
The protons and neutrons are contained in the nucleus, the tiny centre of an atom, with smaller electrons orbiting outside the nucleus.
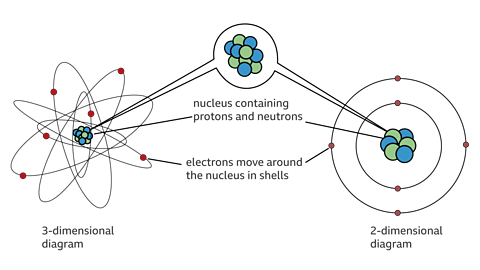
Each particle has its own charge and its own mass.
| Relative electric charge | Relative mass | |
|---|---|---|
| Proton | +1 | 1 |
| Neutron | 0 (neutral) | 1 |
| Electron | -1 | 1/1840 |
The mass of an electron is very small compared to a proton or a neutron. 1 840 electrons have the same mass as 1 proton or neutron.
Since the nucleus only contains protons and neutrons, most of the mass of an atom is concentrated in its nucleus.
Protons and electrons have electrical charges that are equal and opposite. An atom is neutral because it always contains the same number of protons and electrons.
Remember: Proton Positive Neutron Neutral
Size of an atom
Atoms are very, very small. They are too small to be seen, but imaging of atoms by really powerful microscopes is possible. Despite their small size, scientists have managed to find out a great deal about them.
- atoms have a radius of about 0.1 nm (1 û \(10^{-10}\) m)
- the nucleus is less than 1/10 000 of that of the atom (less than 1 û \(10^{-14}\) m)
For comparison, the radius of a typical human hair is about 1 û \(10^{-4}\) m.
If you could count all of atoms in a dot of ink you would find that the number is greater than the population of the world.
Atomic number and mass number
Oxygen and carbon are both elements. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. But what makes oxygen different from carbon?
It is the number of protons in the nucleus of an atom that is the defining feature of the atom. It's what makes one element different from another. The number of protons in an atom is called its atomic number. The atoms of each element in the periodic table have different number of protons, and hence different atomic number. In fact, the periodic table is arranged in order of increasing atomic number.
The atomic number of an atom is the number of protons in its nucleus
- all the atoms of a given element have the same atomic number
- the atomic number of each element is unique - no two elements have the same atomic number
For example,
the atomic number of carbon is 6
This means that:
- there are 6 protons in the nucleus of every carbon atom
- no other element has atomic number 6
the atomic number of oxygen is 8
This means that:
- there are 8 protons in the nucleus of every oxygen atom
- no other element has atomic number 8
A second number is needed to fully describe an atom. This number is called the mass number. Since the mass of an atom comes almost entirely from the protons and neutrons, it is fairly obvious that the mass number is the total number of protons and neutrons
The mass number of an atom is the total number of protons and neutrons in its nucleus
mass number = number of protons + number of neutrons
If you know the mass number (number of protons + number of neutrons) and the atomic number (number of protons), then you can calculate the number of neutrons:
number of neutrons = mass number ã atomic number
Summary
atomic number = number of protons in nucleus
- mass number = number of protons + neutrons in nucleus
- number of neutrons in nucleus = mass number - atomic number
Since an atom is neutral there are the same number of protons and electrons. So, the atomic number also equals the number of electrons orbiting the nucleus. But remember, there are no electrons inside the nucleus.
Calculating the number of protons, neutrons and electrons in an atom
When we write the symbol for an atom, we can place its mass number at the top left and its atomic number at the bottom left.One way to remember the order of these numbers is the word ma: mass number then atomic number
This is the chemical symbol for sodium
ã Mass number = 23ã Atomic number = 11
Number of protons in a sodium nucleus = 11 (the atomic number)Number of electrons orbiting a sodium nucleus = 11. (the atom is neutral)Number of neutrons in a sodium nucleus = 23 ã 11 = 12. (mass number - atomic number)
Question

This is the chemical symbol for chlorine, written with its mass number and atomic number. Calculate the number of protons, neutrons and electrons an atom of chlorine contains.
- Mass number = 35
- Atomic number = 17
Number of protons in a chlorine nucleus = 17
Number of electrons orbiting a sodium nucleus = 17
Number of neutrons in a sodium nucleus = 35 ã 17 = 18
Question

How many protons does Carbon contain?
- Atomic number = 6Number of protons in the carbon nucleus = 6
Question
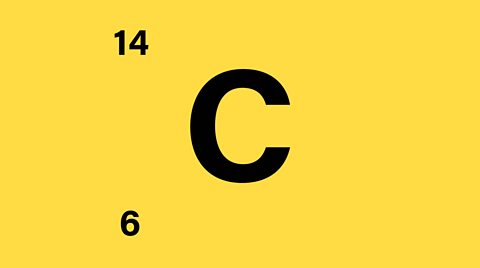
How many neutrons does Carbon contain?
- Mass number = 14
- Atomic number = 6
Number of protons in a carbon nucleus = 6
Number of protons + neutrons = 14
Number of neutrons in this carbon nucleus = 14 ã 6 = 8
Write down the number of particles in each of the following atoms:
Question
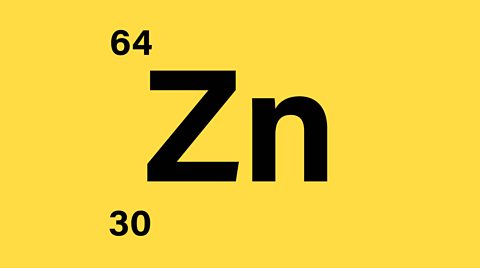
- Number of protons = 30
- Number of protons + neutrons = 64
- Number of neutrons = 64 - 30 = 34
- Since the atom is neutral there are 30 electrons orbiting the nucleus
There are 30 protons, 30 electrons, and 34 neutrons.
Question

- Number of protons = 92
- Number of protons + neutrons = 238
- Number of neutrons = 238 - 92 = 146
- Since the atom is neutral there are 92 electrons orbiting the nucleus
There are 92 protons, 92 electrons, and 146 neutrons.
Question
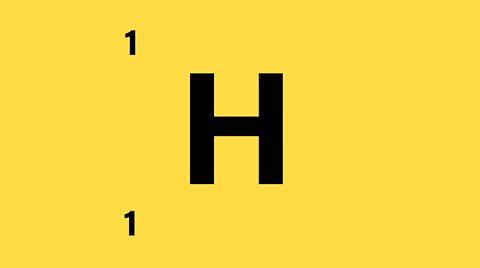
ã Number of protons = 1
ã Number of protons + neutrons = 1
ã Number of neutrons = 1 - 1 = 0
ã Since the atom is neutral there is 1 electron orbiting the nucleus
There is 1 proton, 1 electron, and 0 neutrons.
Question
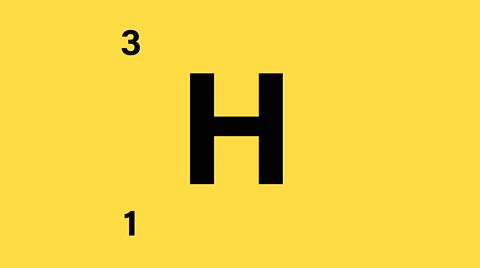
- Number of protons = 1
- Number of protons + neutrons = 3
- Number of neutrons = 3 - 1 = 2
- Since the atom is neutral there is 1 electron orbiting the nucleus
There is 1 proton, 1 electron, and 2 neutrons.
Be careful of a question like the one below!
Question

Write down the number of particles in a nucleus of aluminium, Al
- Number of protons = 13
- Number of protons + neutrons = 27
- Number of neutrons = 27 - 13 = 14
- Since this is a nucleus there are no electrons!!!
There are 13 protons and 14 neutrons in the nucleus of an Aluminium atom.
Always check: is the question asking about the number of particles in the atom (in which case you include the number of electrons), or is it asking about the number of particles in the nucleus (in which case there are no electrons)?
Question
An Argon atom has 18 electrons and 22 neutrons. Write the chemical symbol argon, Ar, with its mass number and atomic number
- Number of electrons = 18 and so number of protons = 18. Hence atomic number = 18
- Number of protons + neutrons = 18 + 22 = 40. Hence mass number = 40The chemical symbol for argon with its mass number and atomic number is:

Isotopes
Isotopes are atoms of an element with the same atomic number but a different mass number, indicating a different number of neutrons.
How electrons are arranged ã The electronic structure of elements
Electrons orbit the nucleus in shells. There can be several shells and:
- each shell is at a different distance from the nucleus
- the first shell is nearest to the nucleus
- different shells hold a different maximum number of electrons
- electrons completely fill up the lowest available shell first ã i.e., the one nearest the nucleus
- when this shell is full, the electrons begin to fill the next shell, and so on
The table below shows the maximum number of electrons for each of the first three shells
| Shell | Maximum number of electrons |
|---|---|
| First | 2 |
| Second | 8 |
| Third | 8 |
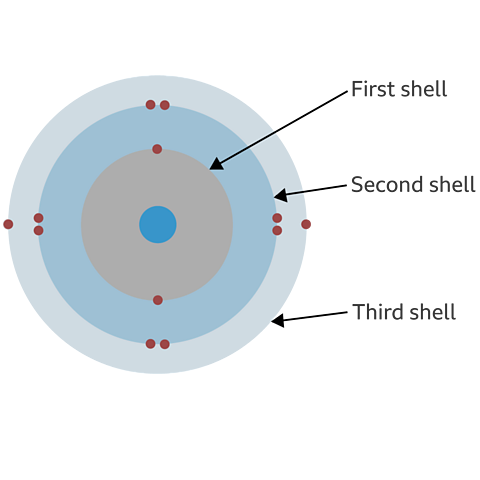
The electronic configuration or structure of an atom is how the electrons are arranged as they orbit the nucleus. Electronic structure can be shown as a list of numbers or as a diagram.
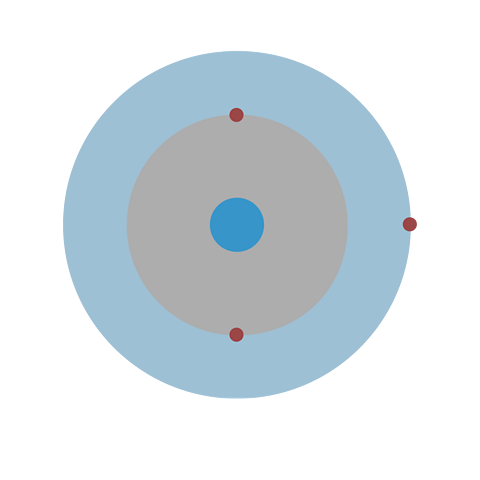
Take lithium for example. The diagram above shows each shell as a circle around the nucleus, with each of the three electrons represented by a dot.
The electronic structure for lithium is written as a list of numbers separated by commas is 2,1. This shows that lithium atoms have three electrons, two in the first shell and one in the second shell.
Sodium has 11 electrons. These will be arranged as:
- 2 electrons in the first shell
- 8 electrons in the second shell
- 1 electron in the third shellYou can write sodiumãs electronic structure with as: 2, 8, 1
The electronic structure of sodium can also be shown in a diagram. Each dot represents an electron.
Question
Aluminium has 13 electrons. Write the electronic structure as a list of numbers
The first shell will fill up with 2 electronsThe second shell will fill up with 8 electronsThe remaining 3 electrons will go into the third shell
Aluminiumãs electronic structure is: 2, 8, 3
Question
Oxygen has atomic number 8, What is its electronic structure?
Since the atomic number of oxygen is 8, it will have 8 protons in the nucleus. An atom of oxygen is neutral and so will have 8 electrons.
Two electrons will fill the first shell.The remaining 6 electrons will go into the second shell.
The electronic structure of oxygen is: 2, 6.
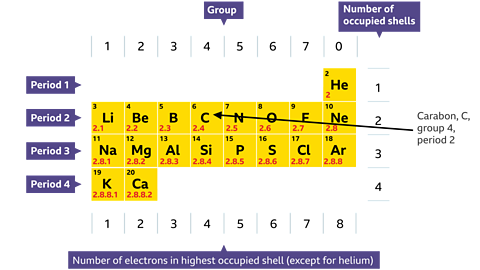
Electronic structure and the periodic table
There are some very interesting links between electronic structure and the periodic table.You will remember from the Periodic Table study guide that the vertical columns in the periodic table are called groups. The groups are numbered from left to right. Elements in the same group have similar chemical properties.
- The number of electrons in the outer shell of all the elements in a group is the same as the group number.
- The number of electron shells of all the elements in a period is the same as the period number.
Examples
All the Group 1 elements - have one electron in the outer shell.
The Group 7 elements - have seven electrons in the outer shell.Group 0 elements have full outer shells.The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell.
Similarly, the atoms of all group 7 elements have similar chemical properties and reactions to each other because all of them have seven electrons in their outer shell.
The arrangement of electrons in the periodic table: /bitesize/clips/zdksb9q
Question
The electronic configuration of carbon is 2, 4. Write down the group number, period number, and atomic number of carbon, C
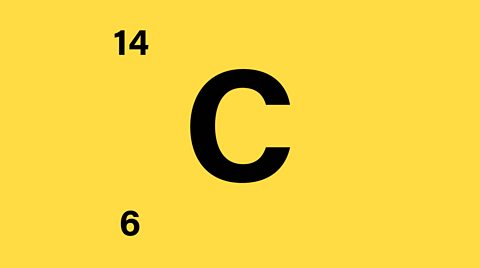
The electronic configuration of carbon is 2, 4. This shows that carbon, C:
- is in group 4 (four electrons in outer shell)
- is in period 2 (two shells occupied)
- has an atomic number of (2 + 4) = 6
Chemical formulae
Remember that we use chemical symbols to stand for the elements. For example, C stands for carbon, O stands for oxygen, S stands for sulfur and Na stands for sodium.
For a molecule, we use the chemical symbols of the atoms it contains to write down its formula. For example, the formula for carbon monoxide is CO. It tells you that each molecule of carbon monoxide consists of one carbon atom joined to one oxygen atom.
Take care when writing your symbols and formulae. Be careful about when to use capital letters. For example, CO means a molecule of carbon monoxide but Co is the symbol for the element cobalt.
Formula and formulae
The word ãformulaeã is the plural of ãformulaã. If we have more than one formula, we donãt say formulas, we say formulae.
Numbers in formulae
We use numbers to show when a molecule contains more than one atom of an element. The numbers are written below the element symbol, as a subscript.
For example, \(CO_2\) is the formula for carbon dioxide. It tells you that each molecule has one carbon atom and two oxygen atoms.
Take care when writing these formulae. The small numbers go at the bottom. For example, \(CO_2\) is correct, but CO2 is wrong.
Some formulae are more complicated. For example, the formula for sodium sulfate is \(Na_2\)\(SO_4\). It tells you that sodium sulfate contains two sodium atoms (\(Na_2\)), one sulfur atom (S) and four oxygen atoms (\(O_4\)).
The formula for a substance is always the same
Remember: A compound is two or more elements chemically combined.
All compounds have a definite composition. For example, a water molecule always contains two hydrogen atoms and one oxygen atom. It cannot be a water molecule if it has different numbers of these atoms. The formula for water is always \(H_2\)O.
Simple compound names
How compounds can be represented by chemical formulae.
The table below shows some common compounds. Itãs useful to know that mono means one, and di means two.
- carbon monoxide has one atom of oxygen
- carbon dioxide has two atoms of oxygen
| Compound | Formula | Elements present |
|---|---|---|
| water | \(H_2\)O | hydrogen, oxygen |
| carbon monoxide | CO | carbon, oxygen |
| carbon dioxide | \(CO_2\) | carbon, oxygen |
| sulfur dioxide | \(SO_2\) | sulfur, oxygen |
| sulfur trioxide | \(SO_3\) | sulfur, oxygen |
| nitrogen monoxide | NO | nitrogen, oxygen |
| nitrogen dioxide | \(NO_2\) | nitrogen, oxygen |
| ammonia | \(NH_3\) | nitrogen, hydrogen |
| hydrochloric acid | HCl | hydrogen, chlorine |
| nitric acid | \(HNO_3\) | hydrogen, nitrogen, oxygen |
| sulfuric acid | \(H_2\)\(SO_4\) | hydrogen, sulfur, oxygen |
If a compound is made from a metal and a non-metal:
- the metal forms the first part of the compound, the non-metal forms the second part
- the ending of the non-metal is replaced with -ide if the compound is made of two elements, and by -ate if it is made of three elements, one of which is oxygen
For example:
Carbon dioxide, \(CO_2\) (ending of oxygen replaced by -ide as there are two elements)
Magnesium Iodide, \(MgI_2\) (ending of iodine replaced by -ide as there are two elements)
Copper (II) sulfate, \(CuSO_4\) (ending of sulfur replaced by -ate as it is made of copper, sulfur and oxygen)
Magnesium carbonate, \(MgCO_3\) (ending of carbon replaced by -ate as it is made of magnesium, carbon and oxygen
Chemical formulae for simple metal compounds - valency
So, how are the numbers in simple formulae worked out?
When writing formulae for simple metal + non-metal compounds, the valency of the elements must be considered.
Valency
Valency is the ability of an atom to gain or lose electrons. The valency of an element is linked to how many electrons an atom must gain or lose to achieve the same electronic structure as a noble gas i.e., have a full outer shell.
For example
an element in group 2 has two electrons in its outer shell. The simplest way to have a full outer shell is to lose two electrons, and so the valency is 2
an element in group 5 has five electrons in the outer shell. The simplest way to have a full outer shell is to gain three electrons, and so the valency is 3
A quick way of calculating the valence of an atom is:
- For Groups 1 ã 4 the valency is the same as the group number
- For Groups 5 ã 7 the valency is 8 minus the group number
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 0 |
| Valency | 1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
Sometimes a Roman numeral is used to tell you the valencyFe(III) means that iron has a valency of 3
Use the periodic table and the rules above to check the valency of each of the following elements
| Element | Symbol | Group | Valency |
|---|---|---|---|
| potassium | K | 1 | 1 |
| magnesium | Mg | 2 | 2 |
| bromine | Br | 7 | 1 |
| nitrogen | N | 5 | 3 |
| carbon | C | 4 | 4 |
| calcium | Ca | 2 | 2 |
| sulfur | S | 6 | 2 |
| copper(II) | Cu(II) | - | 2 |
| silver(I) | Ag(I) | - | 1 |
To write the chemical formula for a compound you can use the S.V.S.D.F system.
- S - write down the symbols of both the elements involved.
- V - beneath each symbol, write itãs valency. Memorise the rules above.
- S - swap the valences over.
- D - if the valences can be simplified, divide them both by the smaller of the two numbers.
- F - write the formula.
(A mnemonic to remember this is: Study Valency to Simply Draw-up Formula)
Example
What is the formula for sodium chloride?
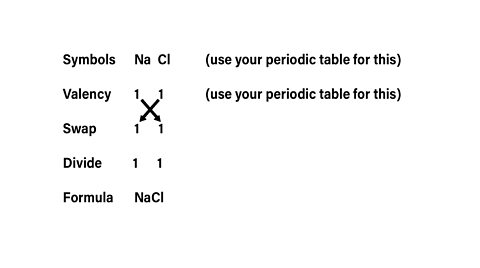
The formula for sodium chloride is NaCl
Example
What is the formula for water if the elements involved are hydrogen and oxygen?
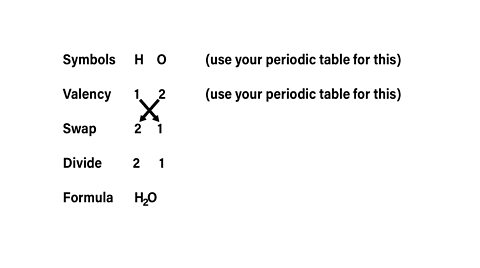
The formula for water is \(H_2\)O
Example
What is the formula for carbon dioxide?
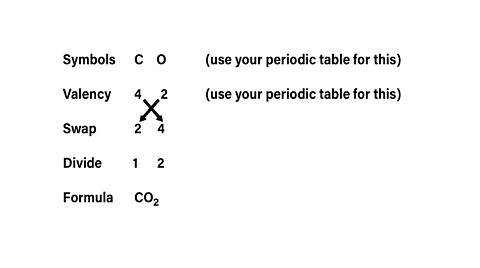
The formula for carbon dioxide is \(CO_2\)
Now try these for yourself:
Example
Question
What is the formula for potassium oxide?
Question
What is the formula for aluminium oxide?
Question
What is the formula for carbon sulfide?
Formula using roman numerals
Some elements, particularly the transition metals, do not always have the same valency in their different compounds. The valency of these elements is usually given in roman numerals inside brackets.
| Roman Numeral | Number |
|---|---|
| I | One |
| II | Two |
| III | Three |
| IV | Four |
| V | Five |
| VI | Six |
Example
What is the formula of copper(I) oxide?
Try this one for yourself:
Question
What is the formula of Iron(III) sulfide?
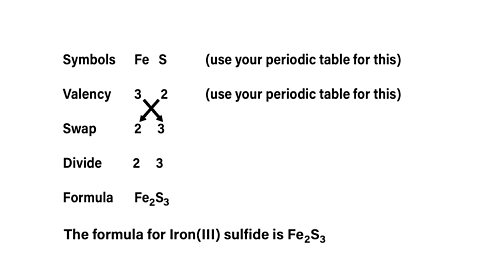
It is worth noting that that valency will be given on the back of the Periodic table for transition metals.
More on Chemistry
Find out more by working through a topic
- count6 of 8

- count7 of 8

- count8 of 8

- count1 of 8
